A single dose of the Oxford/AstraZeneca coronavirus vaccine is 76 per cent effective from three weeks to 12 weeks after the injection, according to an analysis of trial data that could boost confidence in the UK’s decision to delay second doses and vaccinate more people more quickly.
In a paper published on Tuesday but not yet peer-reviewed, researchers at Oxford university said the single dose had shown efficacy of 76 per cent from 22 up to 90 days post-vaccination, “with the protection showing little evidence of waning in this period”.
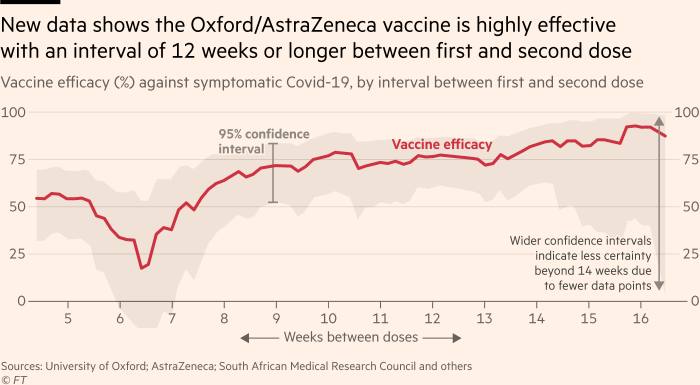
The researchers also found that spacing the first and second doses over a longer period yielded better long-term efficacy — a fact previously defended theoretically, but not shown empirically with large-scale evidence for coronavirus vaccines. Vaccine efficacy rose to 82.4 per cent following a second doses after 12 weeks, Oxford said.
Additionally, the vaccine may have a significant impact on transmission, Oxford said, after it reduced the number of positive coronavirus tests among those vaccinated by 67 per cent. However, a much bigger sample will be needed to reach definitive conclusions on transmission risk, researchers have said.
Oxford’s Andrew Pollard, the chief investigator for the vaccine trial, said the data provided “an important verification of the interim data that was used by more than 25 regulators” in the UK and Europe to give the vaccine an emergency approval.
“It also supports the policy recommendation made by the Joint Committee on Vaccination and Immunisation (JCVI) for a 12-week prime-boost interval, as they look for the optimal approach to rollout, and reassures us that people are protected from 22 days after a single dose of the vaccine,” he said.
The UK approved the Oxford/AstraZeneca vaccine in December, surprising some observers after it chose to space doses for up to 12 weeks in order to stretch limited supplies, a decision that some critics said had no empirical backing.

Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said the data “definitely provide some evidence to suggest that the eventual protection from two doses of this vaccine are not worsened by having a longer than 28 or 42-day period between doses and tend to confirm what had been shown before, that if anything the eventual efficacy was better”.
While these data should not be taken to say that all questions had been answered, “they certainly do not suggest that the JCVI advice on dose-spacing was in any way incorrect for this vaccine”, he said.
The other manufacturers of coronavirus vaccines approved for use in the UK and EU, BioNTech/Pfizer and Moderna, have not tested their vaccines over longer dosing schedules and continue to recommend two doses up to four weeks apart.
But Azra Ghani, chair in infectious disease epidemiology at Imperial College London, struck a cautious note. While it was “tempting to interpret these estimates as indicative of a higher efficacy with a longer dosing gap”, the study was not designed to look at different dosing gaps or at one versus two doses, he pointed out.
“This means that participants weren’t randomised and it is therefore quite possible that there are other things that are driving this apparent trend with dosing schedule,” added Prof Ghani.
Those who received a single dose “were younger, more likely to be female, more likely to be a healthcare worker, more likely to be resident in Brazil and more likely to be white than those who received two doses. In addition, those who received a single dose were followed for a significantly longer period of time,” he added.
Matt Hancock, UK health secretary, described the study as “hugely encouraging” and said it “reinforces our confidence that vaccines are capable of reducing transmission and protecting people from this awful disease”.
https://news.google.com/__i/rss/rd/articles/CBMiP2h0dHBzOi8vd3d3LmZ0LmNvbS9jb250ZW50L2RlMDBmZTc1LTliYTEtNGJlNy04Y2YxLTZlOWZhNTVmMDljZdIBP2h0dHBzOi8vYW1wLmZ0LmNvbS9jb250ZW50L2RlMDBmZTc1LTliYTEtNGJlNy04Y2YxLTZlOWZhNTVmMDljZQ?oc=5
2021-02-02 19:08:00Z
52781349183034
Tidak ada komentar:
Posting Komentar